What happened to the stem cell treatment that was supposed to cure everything?
In the early 2000s, people had huge expectations that stem cell research would bring us wonderful new treatments for a range of diseases. So what happened?
Stem cells are actually quite an incredible concept:
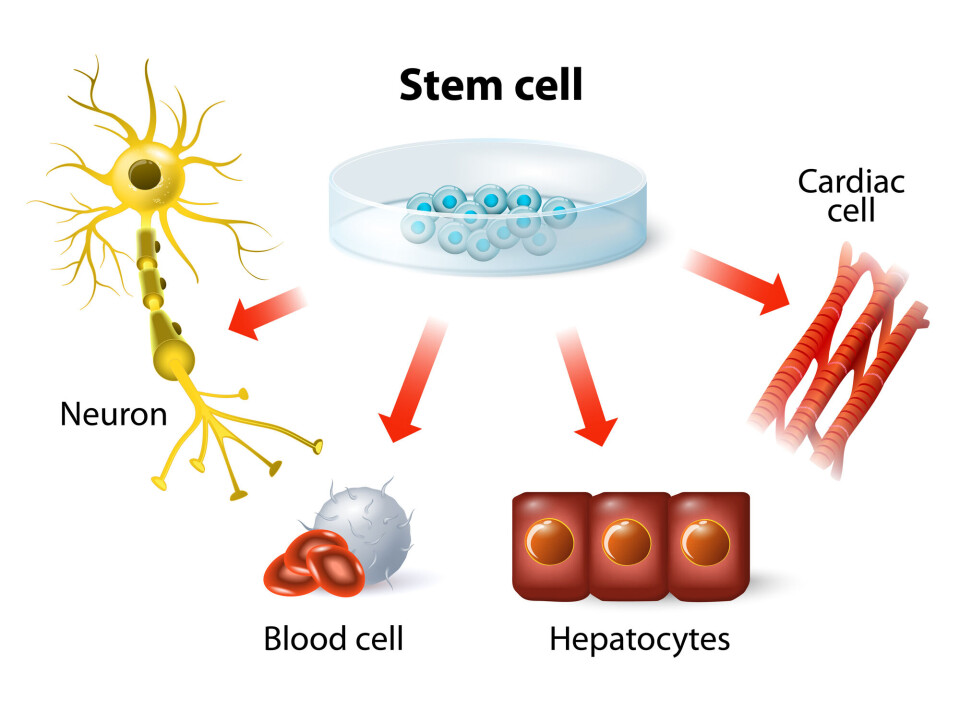
A single type of cell that can develop in many different ways to become a range of specialized cells.
With the help of this very ability, a single fertilized egg cell can make all the cell types needed in a human body: liver cells, heart cells, blood cells, nerve cells and a bunch of others.
In addition to universal stem cells — which can become anything — tissues and organs also contain more specialized stem cells. Bone marrow, for example, contains stem cells that can become all the different types of blood cells. This is how the body replaces cells that die.
Our understanding of stem cells is not entirely new.
Scientists have known since the 1960s that these cells exist, but it was just before the turn of the millennium that things really got going.
Made stem cells from skin
In 1998, a team of researchers managed to isolate stem cells from a human embryo and grow them in the laboratory. This opened up dizzying possibilities.
In theory, it should be possible to use stem cells to grow new cells and tissues – even whole organs.
The idea was to use this tissue to replace damaged cells and organs, thereby curing diseases such as Parkinson's, Alzheimer's, type 1 diabetes, heart attacks and strokes.
The same approach might also perhaps repair spinal cord injuries after accidents, so that people with paralysis could move again.
High hopes
Stem cells soon became a hot topic.
Hope and hype abounded in the media. But the researchers themselves also had great expectations, not least fuelled by some fantastic breakthroughs during the 2000s.
The biggest sensation was the work of the Japanese researcher Shinya Yamanaka.
In 2006, forskning.no wrote about (link in Norwegian) how his team had managed to reprogram ordinary skin cells in mice to become embryonic stem cells, making them the very earliest universal cell that could become any other cell in the body.
A year later, they did the same with human skin cells.
“These were incredible breakthroughs,” says Joel Glover, a professor at the University of Oslo and head of the Norwegian Center for Stem Cell Research.
Until then, it had been thought that development could only go one way — from embryonic stem cells to more and more specialized stem cells that can make fewer and fewer types of cells.
But Yamanaka and his colleagues had reversed this process. Yamanaka received the Nobel Prize for this work just six years later.
Thought new treatments would come quickly
“15 years ago I said in an interview that we would probably have some form of stem cell treatment for spinal cord injury within ten years,” Glover said.
He was not alone in having high hopes.
By the end of the 1990s, researcher and section chief doctor Jan Brinchmann talked to his colleagues at Oslo University Hospital about the fact that they would probably use stem cells on patients very soon. They were given the go-ahead to build a laboratory where it was possible to grow these cells.
“We expected that stem cell treatment would quickly enter the clinic,” he said.
Professor Philippe Collas at the University of Oslo, who has worked with stem cells and cloning for 30 years, said more or less the same thing.
But it didn’t happen quite like that.
Today — 20 years later — there is still no stem cell treatment for spinal cord injuries.
Revolutionary studies that the company Geron initiated towards the end of the 2000s were suddenly stopped.
Projects that were supposed to cure Parkinson's, eye diseases or diabetes also did not deliver the results everyone was hoping for. And people with heart, liver or kidney disease still cannot hope to get transplants of new organs grown from their own stem cells.
What happened?
Complicated biology
“It has turned out to be much more difficult than we thought to turn this into treatment for patients,” Brinchmann said.
As scientists in other fields have experienced in recent decades, stem cell researchers discovered that biology is more complicated than expected.
One of the challenges is that stem cells develop in many stages, from universal stem cells to fully specialized cells such as liver cells or insulin-producing cells.
“In order for stem cells to work optimally, they must be developed to the final stage. But it’s not so easy to get to the last step,” Brinchmann said.
Beta cells that produce insulin are a good example. In people with type 1 diabetes, these important cells are destroyed. Perhaps the damaged cells can be replaced with new beta cells, grown from stem cells?
“Researchers managed to get to the penultimate stage before the cells turned into beta cells, but struggled to get them to the last stage, which is necessary for them to produce the right amount of insulin,” Brinchmann said.
Problems in practice
The same problem has cropped up in liver cell research. Researchers have managed to create cells that are similar to foetal liver cells, but the cells are unable to do all the tasks that are needed.
Nor do the cartilage cells that Brinchmann and his colleagues have worked to develop work as well as natural cartilage.
And even where scientists have managed to create new cells, the road to treatment can be long and difficult. Because how do you get the new cells to the right place?
For example, researchers have thought that new nerve cells could be used to treat ALS, the very serious disease in which the nerve cells that control the muscles die.
But how do you deliver the new cells along the entire spinal cord? Currently, there are no ways to do this.
In addition, there’s another question researchers have to grapple with: Are we sure that stem cell treatment does not cause cancer?
It is known that stem cells that are not fully developed can give rise to cancerous tumours. Before stem cells can be used in new types of treatment, large studies have to be carried out to rule out serious side effects. That’s not always so easy.
Hype, but still hope
Glover, Brinchmann and Collas believe that the storm surrounding stem cells in the early 2000s was hype.
Nevertheless, stem cell research is still important. There is still considerable hope that it will provide new ways of treating disease in the future, they believe.
Slowly but surely, the results are starting to come in.
“Now, after so many years, we are finally seeing the possibility of good results, for example with new bone tissue,” Brinchmann said.
“There is a world-leading research group in Bergen. It has developed a way to build up jawbone with stem cells and a biomaterial, so that the jawbone becomes strong enough to withstand the implantation of teeth,” he said.
“This is the first success story with this type of stem cell, which has been considered promising for 20 years,” he said.
There’s also a lot of international research.
Although researchers have not been able to create whole organs as they had hoped, they have come a long way in creating organoids. These are small organs from cultured cells.
A Dutch group, for example, has come far in the development of intestinal organoids, which can perhaps be transplanted into patients who have Crohn's or other intestinal diseases.
Stem cells are not the solution to everything
At the same time, the researchers' ambitions have perhaps become more realistic.
Collas does not believe that stem cells are necessarily the solution to all the problems we thought the cells could fix 20 years ago. Perhaps this fix will come from other areas of research.
“In the last ten years, we have seen a parallel development in regenerative medicine with robotics and electronics. Perhaps we will not cure paralysis after spinal cord injuries with stem cells, but with electrical connections,” said Collas.
In many ways, the physics of electronics and robotics is easier to understand and control than the incredibly complex systems of biology, he said.
He nevertheless believes that stem cells can play a major role in medical treatment in the future.
“I think tissue that can be transplanted will be developed. This is also promising for disease-specific drug testing,” he said.
Knowledge of the mechanisms
Stem cell technology makes it possible to find the best possible treatment for individual patients, without having to test drugs and make mistakes on the patient, Brinchmann said.
A person with a genetic disease that causes heart rhythm disturbances offers insight into how this might work.
“We can take skin cells from the patient, make immature stem cells from them and grow them in the laboratory. Then we can make heart cells from these stem cells,” he said.
“We can then see that the heart cells have rhythm disturbances and test different medicines until we find the one that works best on this particular disturbance,” Brinchmann said.
Furthermore, the researchers can use these stem cells to understand the mechanisms that lead to the arrhythmias and why they occur in the first place.
This understanding of basic mechanisms in the human body is one of the great benefits of the last 20 years of stem cell research.
Today, we know much more about the processes that control stem cells, such as what causes a certain stem cell to become a bone cell, and not a fat cell or cartilage cell.
“We also understand more about the earliest phases of foetal development,” Glover said.
“This is important, because we think many diseases already have begun by that point. Developmental disorders can occur that predispose a person to illness later in life,” he said.
Organoids, such as the mini-brains the researchers have managed to grow, will eventually give us even more insight into how our body works.
More knowledge about these mechanisms can eventually provide new ways to prevent or treat disease.
“I would say that within the next 20 years we will have several examples of stem cell-created tissues and organs that are in use in the clinic. In 50 years, there may be many examples,” Glover says.
But not whole hearts.
He thinks we will have to wait a long time for that.
Translated by Nancy Bazilchuk
———
Read the Norwegian version of this article at forskning.no
------