THIS CONTENT IS BROUGHT TO YOU BY University of Oslo - read more

Researchers are testing a new product to relieve dry mouth
The hope is that it can relieve dry mouth over a longer period of time.
Researcher Julie Frigaard at the University of Oslo's Institute of Clinical Dentistry, together with Professor Marianne Hiorth at the Department of Pharmacy, developed the mouth spray Liposal. It contains polymer-coated liposomes. This means that long molecules called polymers cover the surface of the liposomes.
Liposomes are tiny spherical particles.
“In the lab, we've tested different ingredients and concentrations in the mouth spray. We've looked at what stays stable, as the product needs to have a certain durability," Frigaard says.
Additionally, the spray must be non-toxic.
She explains that an important task for them has been to test the ingredients on living cells to see if there is anything dangerous or harmful to them.
Liposal or placebo?
“Now, there's a clinical trial of Liposal, and it's a milestone that we've come this far,” Professor Janicke Liaaen Jensen says. She has been Julie Friaard's main adviser on her doctoral project.
Liposal is a special product that, in theory, should adhere to the oral mucosa of patients with dry mouth and slowly release moisture. This way, it is hoped to have a more long-lasting effect than other products on the market.
Liposal is produced in Marianne Hiorth's lab at the Department of Pharmacy. The researchers there have had a significant job producing it and getting it ready and approved before the trial.
They also created the control product in the lab. It contains the same ingredients as Liposal except for the polymer-coated liposomes.
Then they filled two bottles for each patient, one with Liposal and one with the placebo. Only they know what is in the bottles, while Frigaard, Jensen, the research nurse, and the patients are unaware of this.
"Can you patent the product and put it into production?"
“No, unfortunately, we cannot,” Jensen says.
Several studies with polymer-coated liposomes have already been published by Marianne Hiorth. Therefore, the recipe is based on secrecy. For that reason, they cannot disclose what the product contains.
Jensen explains that it is not possible to patent something that has been published previously. Therefore, secrecy is the only way to prevent others from making a similar product.
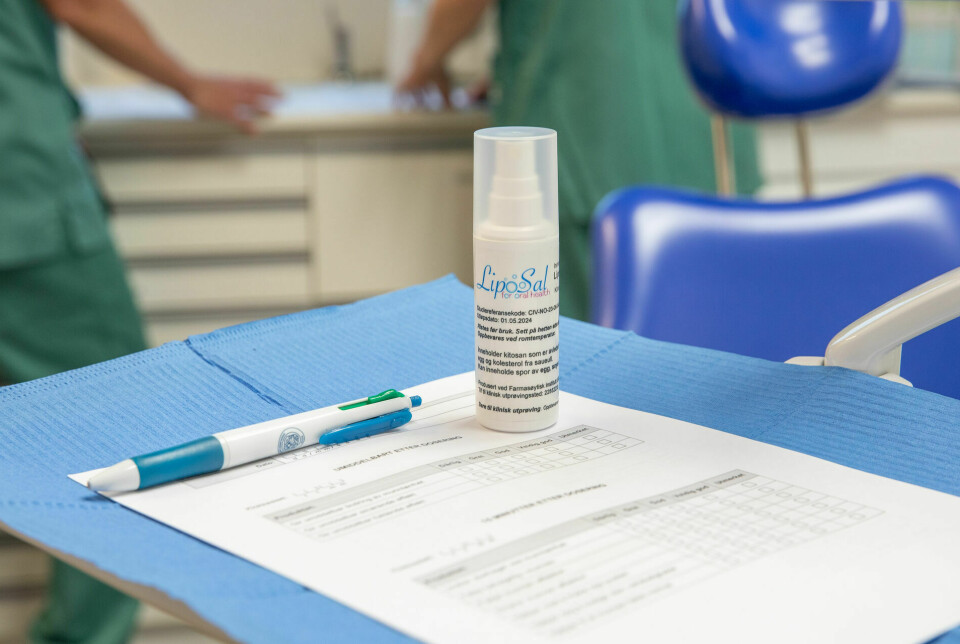
Resource-intensive product testing
Research nurse Carina Athelie Skredlund is employed at Smerud Medical Research. Smerud's role in the project is to ensure that everything is done correctly, such as patient safety and submissions to authorities.
Smerud is a company that supports researchers in projects developing drugs or medical devices.
“Our primary focus is to ensure that we follow the protocol,” Skredlund says.
A protocol in this context is a kind of cookbook that precisely describes everything that needs to be done. Everything must be done exactly as it is written there.
Extensive documentation
20 individuals are included in the trial. They will visit the research clinic three times each.
“There have been some challenges with including patients in the study due to winter weather, slippery conditions, and the presence of influenza, Covid-19, and RSV. Our goal is to complete the study before Easter,” Jensen says.
She further explains that they ensure the patients who are participating meet the inclusion criteria for the study. For instance, they have to have adequate dryness of the mouth and low saliva secretion. This refers to the amount of saliva produced per unit of time.
“There's a substantial amount of paperwork involved. We must document all the patients' illnesses and their onset, as well as all the medications they use and when they began using them. We need to know the dosage, and reasons for use. Thus, there's a comprehensive mapping and documentation of the participating patients,” Jensen says.
Once the patient is cleared, research nurse Skredlund takes over. She goes through how the spray should be used and how the diary should be filled out together with the patient.
The diary contains several questions about the product's usage and effect throughout the day and night. It should be filled out over a whole week.
First, the spray is tested for up to two hours at the research clinic.

Anticipation of the results
The patient is required to spray the contents of the bottle into their mouth three times. Then, they record their perceptions of the spray at that moment and every 15 minutes until the spray no longer has an effect.
"Some participants left after 15 minutes, while others stayed for two hours. Therefore, we hope that those who've stayed for two hours have used Liposal,” Jensen says.
The participants then take the product home for a week and are required to keep a diary, writing both in the morning and evening. They must answer several questions about the product's performance.
"They will maintain a diary for two products and, ultimately, compare and share which they prefer. The most thrilling answer for us is how the participants feel about the product's performance,” she says.
Once all data is collected, Smerud's statistician takes over and summarises the findings in a report.
"This will determine if the product's effects are significant enough to proceed with a larger study, which would examine long-term effects, and side effects, and include additional patient groups. However, if patients express no preference, we will not proceed with this product. Therefore, we are eagerly awaiting the results,” she says.
Homogeneous patient group
The researchers are already familiar with the patient group that is currently testing Liposal. These are patients with Sjögren's syndrome and patients who have been evaluated for Sjögren's before.
Sjøgren's syndrome is a rheumatic disease with chronic inflammation in the salivary and tear glands. This causes the mucous membranes in the mouth and eyes to become dry.
“Many patients we have approached have willingly agreed to participate and are eager to contribute to the trial,” Skredlund says. “These patients should exhibit symptoms such as dry mouth, dry eyes, reduced tear production, and diminished saliva.”
“We have intentionally chosen these patient groups to create a fairly uniform selection. We could also include patients who have undergone radiation therapy for head and neck cancer or patients with dry mouths caused by medication," Jensen says.
These patients will be included by the researchers in a larger study if it turns out that this product is as effective as they hope.
"We need evidence of its effectiveness across multiple patient groups for it to be considered for further development,” Jensen concludes.

This content is paid for and presented by the University of Oslo
This content is created by the University of Oslo's communication staff, who use this platform to communicate science and share results from research with the public. The University of Oslo is one of more than 80 owners of ScienceNorway.no. Read more here.
More content from the University of Oslo:
-
Norway's journey of indigenous language revival: "Their mother tongue was worthless"
-
“Make Sweden Great Again”: The far right found each other on Twitter during the Swedish election
-
Thousands-year-old animal bones discovered in cave: "We've found several species that have surprised us"
-
This is how researchers can detect invisible and odourless gases
-
Norwegian answer to ChatGPT is on its way
-
Cod has been dubbed the chicken of the sea