Norwegian researchers make strides toward diabetes cure
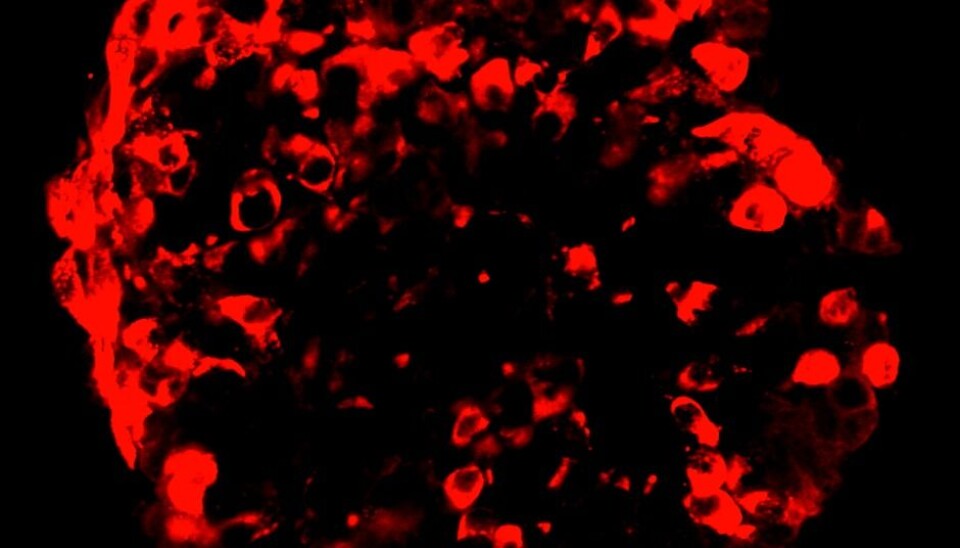
Researchers in Bergen have transformed skin cells from diabetes patients into insulin producing cells. The goal is to transplant them under the skin of persons with the disease.
This is a short-term step toward finding out how to make implanted cells secrete insulin in the body.
Further down the road the goal is to replace insulin injections and blood sugar monitoring with automatic insulin secretion in keeping with blood sugar levels by implanting a capsule containing custom-made cells for each individual diabetes patient.
“This is a step further in ‘spare-part’ medicine, where much can go awry and everything must fall in place before there can be a permanent, reliable healing of diabetes,” explains Helge Ræder, professor of medicine at the University of Bergen (UiB).
The study was newly published in Scientific Reports.
Too few donors
Nearly a century has passed since the first artificial production of insulin, enabling diabetes patients to survive with injections of the pancreatic protein hormone.
No real revolution in diabetes treatment has occurred since. The main treatment is still with insulin substitutes and various methods of injecting insulin.
Diabetes still requires daily follow-ups by the patients themselves. Those with type 1 diabetes have to gauge their blood sugar levels and inject doses of insulin depending on how much they eat, what they eat and how much exercise they get.
A few diabetes patients receive transplanted insulin producing cells from dead donors. There are not enough of these to go around.
“There are simply not enough donors to help everyone,” explains Ræder.
Another challenge is that the cells can be rejected or attacked and destroyed by immune defence mechanisms or could cause disease.
“This is why we are experimenting with the artificial production of insulin producing tissue from the patient which can do the job without mishaps,” says Ræder.
Race to succeed
Medical scientists round the world are dashing to succeed in restoring insulin production within the body with the use of insulin producing cells.
There is an enormous market for a successful treatment – 400 million people worldwide have diabetes.
The researchers in Helge Ræder’s groups at UiB have made an important step toward a cure. According to Ræder they are the leaders in Europe and are competing with American scientists for the lead.
“It isn’t all-important for us to be first, but once a treatment is found we want to contribute toward making it as safe as possible,” says Ræder.
The team in Norway has used skin cells from diabetes patients and re-programmed them to produce insulin. The risk of rejection is lowered because these are the patients’ own cells.
Making stem cells from skin cells
But insulin is produced by so-called beta cells in the pancreas. How can skin cells be altered to do that?
The first step has been to change skin cells into stem cells.
Stem cells can become any type of cell in the body.
In the initial cluster of cells from divisions after fertilisation all the cells are stem cells until they are genetically ordered to become nerve cells, intestinal cells and so on, a wide variety including pancreatic beta cells that produce insulin.
The Bergen team has used the technology developed by the Japanese scientist Shinya Yamanaka that won him the Nobel Prize in Medicine in 2012. It involves reverting cells back into the stage where they were stem cells.
“We have imitated the embryonic process by adding chemical compounds to make the cells produce insulin,” explains Ræder.
This has enabled he and his colleagues to avert the use of stem cells from aborted embryos, which for some people poses an ethical dilemma.
Secrets of secretion
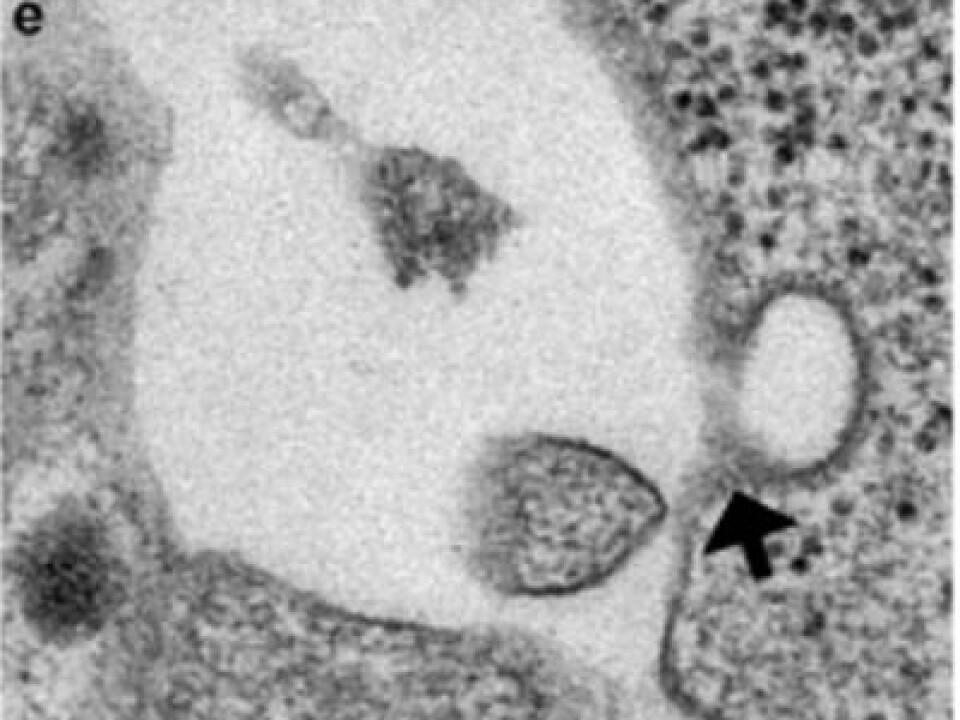
The cells do produce insulin, as they have for similar research in the USA. There are still many pieces of the puzzle to fit in.
How do you get the cells to secrete insulin when the body needs it?
“Well, that’s the crux of the matter. Secretion mechanisms pose the big challenge,” says Ræder.
So far the researchers have coaxed the cells into secreting insulin by adding potassium chloride. But glucose is what triggers the body’s production naturally and that is the target compound. The University of Bergen researchers use leftover bits of cells from the pancreases of dead donors. This material would otherwise go to waste.
Here is where they can find the signal substances that trigger insulin production.
“This has been approved of by ethics committees,” stresses Ræder.
Implants in five or ten years?
Plenty of research is needed before scientists get control of the quantities of insulin secreted according to need, so that this regulation is automatic as it is in healthy persons.
But it is hard to get cells to secrete the right doses of insulin according to glucose levels.
The group of researchers in Bergen are now doing some experiments with such cells in mice.
When will these artificial cells become available for diabetes patients?
“I would guess that this might be within five or ten years. But it could also be as close off as two years or as far ahead as 20 years. It’s impossible to say,” discloses Ræder.
Functioning capsules
The next challenge is to create capsules for such cells for implantation. These would send insulin into the bloodstream while keeping the cells in place and prevent them from disappearing into the body.
The scientists have to stop the growth of such cells when they are just mature enough to produce insulin. Mature beta cells normally lose the ability to grow.
“For safety sake we have to ensure that the cells stay within the capsule. Otherwise, worst case, they can cause cancer in the patients if they keep growing and have escaped the bounds of the capsule,” warns Ræder.
He thinks the American researchers might be moving too quickly as they have already started studies involving patients.
The research group in Bergen is cooperating with the Norwegian University of Science and Technology (NTNU) in developing capsules that work optimally. Another challenge is to prevent the tissue in contact with the capsule from becoming so dense and hard that it prevents the insulin from passing into the body.
“This needs to be safe before we start experimenting on people,” he stresses.
One piece of the puzzle
Sven Magnus Carlsen, professor at NTNU’s Faculty of Medicine and Health Sciences suggests the current progress is just a tiny piece of a very large puzzle. He has conducted research on diabetes for years.
“They haven’t managed to make the cells release insulin into the body. That is the first step. Then they need to get the insulin to release in the right doses corresponding with the glucose levels in the body,” he commented to the Norwegian Broadcasting Corporation’s local branch office NRK Hordaland.
Ræder’s research group has been received research funds from the Bergen Research Foundation in addition to financial help from the University of Bergen, Novo Nordisk Foundation and the Norwegian Diabetes Association.
-------------------------------------
Read the Norwegian version of this article at forskning.no
Translated by: Glenn Ostling
































