Some have started using an alternative to fluoride in toothpaste. How does it work?
The mineral appears to have some beneficial effects that fluoride does not. However, two Norwegian researchers still trust fluoride more.
For more than 50 years, fluoride has elevated Norwegian dental health to new heights.
Fluoride is a mineral in solid form.
In very large doses, fluoride can be toxic. A journalist in The Atlantic writes that she has stopped using toothpaste containing fluoride.
She has started using a new type of toothpaste. It contains the substance hydroxyapatite. How does it compare to fluoride? And should we all consider it - for the sake of our health?
"More and more talk about this"
At the Nordic Institute of Dental Materials (NIOM), researchers regularly receive questions from oral health professionals about this alternative to fluoride toothpaste, according to professor and senior researcher Aida Mulic.

Julie Marie Haabeth Brox also notices increased interest. She is doctoral research fellow at the University of Oslo's Institute of Oral Biology.
"There's more and more talk about this, especially among those who think a bit alternatively," says Brox.
The mineral is the same as that which makes up our tooth enamel. Hydroxyapatite is composed of two minerals: calcium carbonate and calcium phosphate.
The mineral consists of tiny crystals. A complex system of prisms and rods makes enamel the hardest material in the body, Mulic explains.
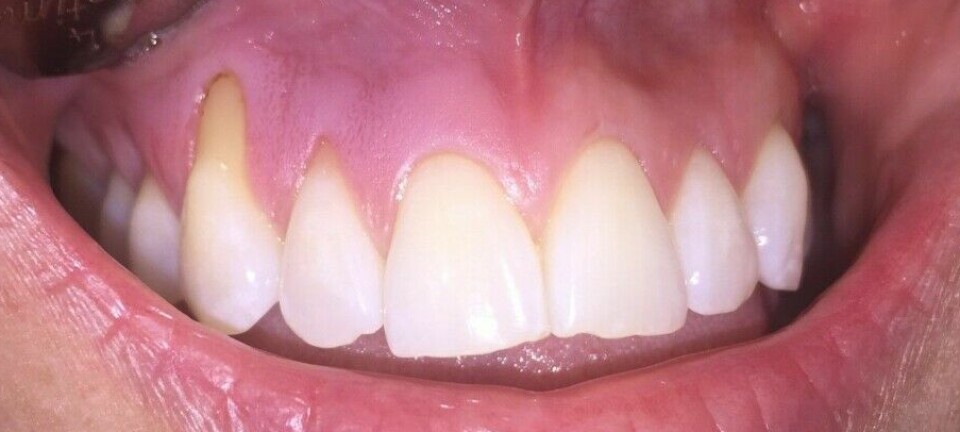
Despite its strength, enamel has one significant weakness.
Enamel breaks down
Acid is enamel's worst enemy. Acid is produced by bacteria when we eat sugar and is also introduced directly through food and beverages, like soda.

Acid causes the minerals in enamel to begin breaking down.
The crystal structure weakens and becomes more irregular. Over time, it becomes thinner.
And this is where fluoride comes in.
We know a lot about fluoride
"Fluoride has a very strong preventive effect against cavities," says Mulic.
Fluoride binds to the crystals. Simply put, it makes the crystal structure stronger and more resistant to acid, preventing it from breaking down so easily.
"The advantage of fluoride is that we know the exact concentration for maximum effect and minimal side effects," says Mulic.
Fluoride has been widely used for decades. In Norway, it became common practice in 1971, marking a phenomenal development in dental health.
With hydroxyapatite, however, there is still much we do not know.
"Promising results"
The idea behind hydroxyapatite toothpaste is similar to that of fluoride.
Research has shown promising results, according to Mulic.

Studies have shown that brushing with this type of toothpaste allows new hydroxyapatite crystals to bond with the enamel. They repair small damages in the structure.
Brox explains that hydroxyapatite dissolves quickly in saliva, releasing a large amount of calcium. This enables the enamel to absorb new crystals into its network.
"This is promising and appears to have a good preventive effect against cavities," says Mulic.
However, she remains uncertain about its overall value.
Low cavity rates in Norway
"Do we need this alternative when we know that fluoride is effective?" she asks.
Mulic expresses some concern about marketing that might lead more people to become sceptical of fluoride.
"There are forces in the media that often emphasise the potential harm of fluoride," Brox points out.
She refers to a high-profile case in the USA where authorities found indications that children in states with high fluoride levels in drinking water may have slightly lower IQs.
Norway recently banned the use of fluorinated compounds in ski wax. This makes people question the safety of putting fluoride in their mouths, says Brox.
Poisoning requires unrealistically large quantities
Both Brox and Mulic emphasise that fluoride is not toxic nor harmful in the concentrations found in toothpaste, tablets, and mouthwash. Fluoride supplements beyond toothpaste are only recommended for patients who are particularly vulnerable to cavities.
That said, children exposed to excessive fluoride during tooth development may develop white spots on their teeth.
"In Norway, this issue is rare," says Mulic.
Fluoride is not added to drinking water in Norway. As a result, it is difficult to consume too much fluoride as long as the recommended amounts in products are followed, she explains.
There are also no reports of chronic poisoning from long-term exposure to harmful levels. It would require unrealistically large quantities, she says.
A potentially good alternative
Fluoride can also repair small damages and wear on enamel, Mulic explains.
However, hydroxyapatite appears to have some benefits that fluoride does not.
"Some studies have shown that hydroxyapatite reduces plaque formation. Others suggest it has a whitening effect, making teeth slightly brighter. These properties are not present in fluoride," she says.
Brox adds that some whitening fluoride toothpastes on the market today contain abrasive agents that wear down enamel.
While Mulic is not opposed to hydroxyapatite itself, she stresses the need for further research.
Fluoride remains the top recommendation
"I believe this toothpaste can be recommended for those who are anxious about fluoride. It may be a good alternative and likely better than nothing," says Mulic.
"However, my recommendation remains brushing twice daily for two minutes with fluoride toothpaste," she adds.
Brox is interested in conducting her own research on good alternatives to fluoride. So far, she believes the knowledge about hydroxyapatite is too limited.
"Hydroxyapatite might be exactly what we're looking for, but we need more research to support it," she says.
Currently, there is no substance that can replace fluoride, Brox emphasises.
First product approved in Europe
Among dentists, hydroxyapatite is still relatively unknown, says Brox. However, something significant happened in March 2023 that might change this.
The first toothpaste with nano-hydroxyapatite was approved in Europe. This was decided by the Scientific Committee on Consumer Safety (SCCS), an independent committee that took several years to approve the product. The toothpaste is made by the company FLUIDINOVA.
"They produce hydroxyapatite in the form that has been shown to work best," says Brox.
This involves nanocrystals, hence the term nano-hydroxyapatite. There are also non-approved products on the market.
These may contain larger crystals, which research has shown to be less effective, Brox says.
———
Translated by Alette Bjordal Gjellesvik
Read the Norwegian version of this article on forskning.no
Related content:
Subscribe to our newsletter
The latest news from Science Norway, sent twice a week and completely free.