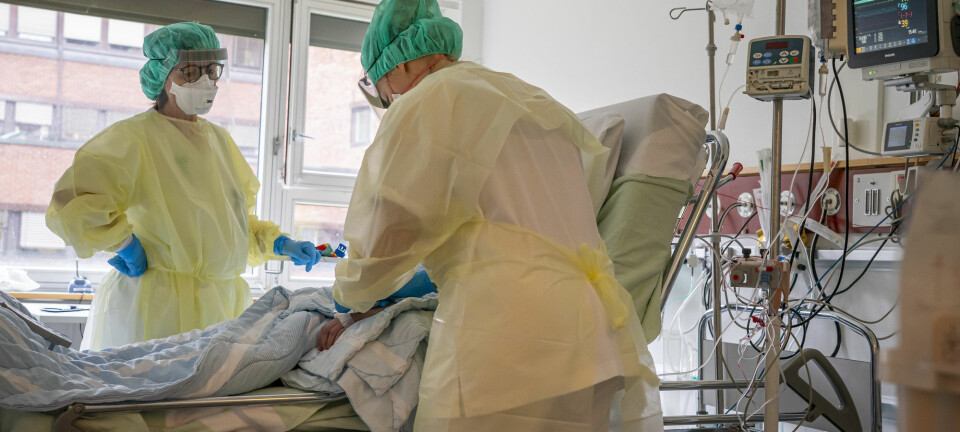
The story of when Norway ran out of coronavirus tests, and a university started a factory to make them
The Nanoparticles had been under development for several years. The bureaucracy was on speed. The researchers were given everything they needed. And then they started a factory.
St. Olavs Hospital in Trondheim was about to run out of coronavirus tests. Norway had been shut down for a week, infection numbers were climbing, but suppliers had no tests left and would not be able to supply them for at least another couple of months.
On March 20, the hospital sent out an email to a range of recipients next door, at the Norwegian University of Science and Technology (NTNU). Maybe some of the researchers or laboratories had some test kits that they could spare?
"There was a sense of emergency," says Magnar Bjørås. "They saw that the infection numbers were increasing."
Professor Bjørås heads a research group at the Department of Clinical and Molecular Medicine at NTNU's Faculty of Medicine and Health Sciences.
He did have some test kits lying around. But not enough to make a difference.
So he decided he had to try and make a new one instead.
We could have run out
February and March feel like a long time ago.
Norway's first coronavirus infection was detected on 26 February. At that time, newspapers warned of the lack of infection control equipment and tests. The criteria for testing were strict and controversial.
"If we test without restrictions and without good reason, we can run out of tests," Frode Forland, from the Norwegian Institute of Public Health (NIPH), told the national newspaper VG on March 7. On April 4, the newspaper Dagbladet published an article with the dramatic title: "Test capacity can keep Norway closed down."
Until then, Norway had been at the top worldwide when it came to testing, despite the lack of testing equipment. But now the NIPH had tightened testing criteria, Forland told Dagbladet, as they struggled to test all the people who wished to be tested. There weren’t enough personnel to perform tests, there weren’t enough machines, and not least, there simply weren’t enough reagents.
The whole world was in fact on the hunt for reagents, which are the chemicals used in coronavirus tests that detect whether a person is infected or not.

We only do research
First, Bjørås created a test that used alcohol to extract viral RNA. It worked, but would be too cumbersome to perform on a large scale. But Bjørås and his research team knew about the nanoparticle research at NTNU, and thought that the magnetic nanoparticles might work for the test.
So the professor contacted the nanoparticle researchers, and postdoctoral fellow Sulalit Bandyopadhyay jumped right in.
"We have been working with these nanoparticles for several years", Bandyopadhyay says by phone from Trondheim. His one-year old and four-year old can be heard playing in the background. The children are finally getting to spend a little bit of time with dad, after an extremely hectic spring.
The nanoparticles that Bandyopadhyay makes can be used in medicines to help them work more precisely in the body, among other uses. Usually this is the stuff of basic research: How do these particles work, and what can they be used for?
"We had some particles in store, but not a lot of them, because we only do research. So my first big challenge was how to make lots of them, so they could actually be used for large-scale testing", he says.
Bandyopadhyay and a PhD student made some fresh batches of nanoparticles and sent them over to Bjørås and his gang.

It took a week
Over the next few days, researchers from across different disciplines worked around the clock. Chemists, biologists, molecular biologists and virologists took breaks just to sleep. After three days, they had promising results. And one week after St. Olavs Hospital reached out, doctors received the new NTNU test.
"It was a success, right away", says Bjørås.
The hospital now compared the new test to the commercial tests they still had in stock. Suspected COVID-19 cases were tested with both the NTNU test and a commercial test. The results showed that NTNU's new test was slightly more sensitive.
"Then I thought, okay, now I am done, and it will be good to take a little time off’", Bjørås said.
But the news spread quickly. Soon, Bjørås was in meetings with the health authorities and the Norwegian Institute of Public Health.
They asked the researchers to produce their test for the whole country. The order was for 5.1 million tests over the next 17 weeks.
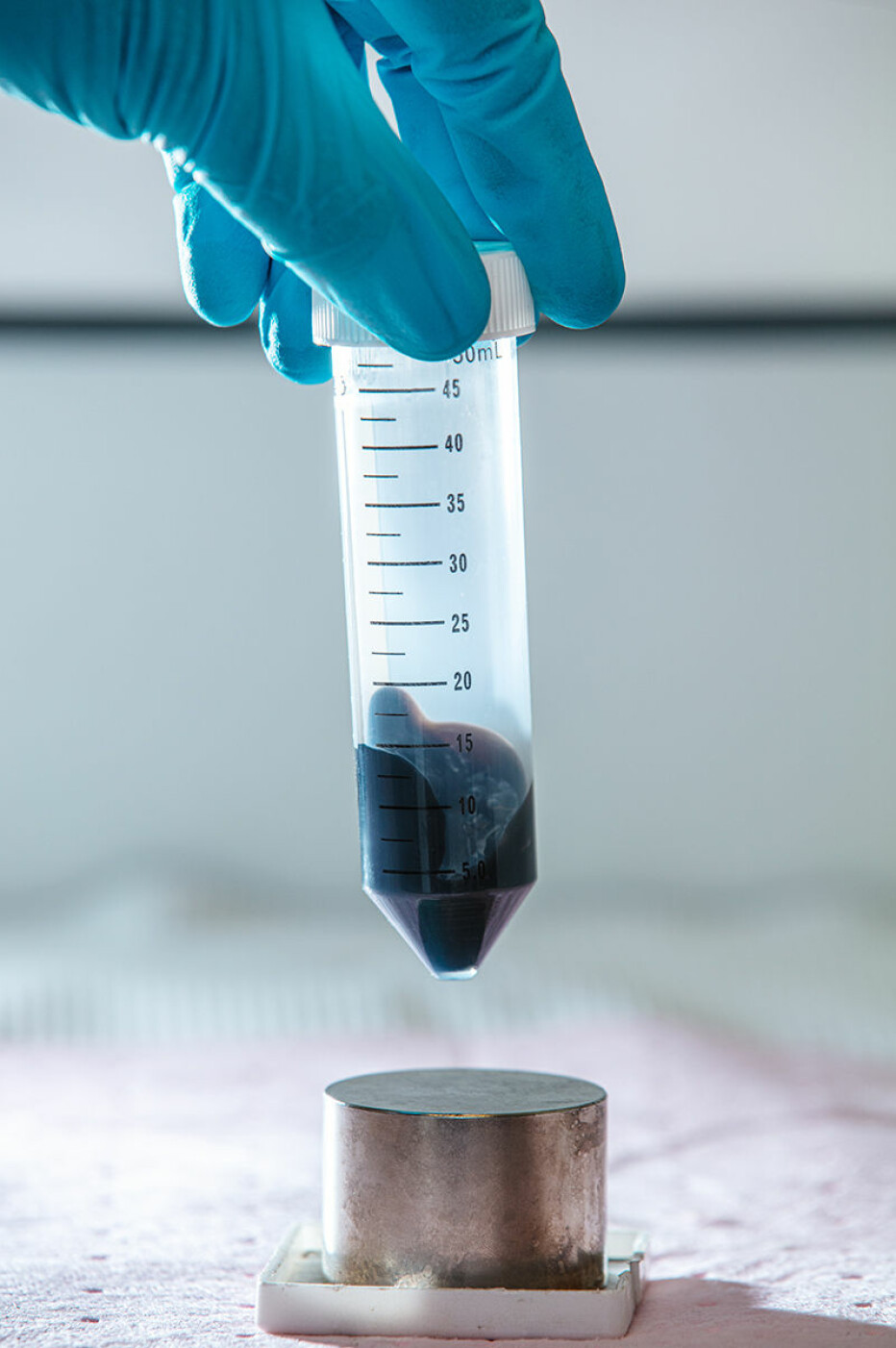
The university factory
The test worked, without a doubt.
But how could they make millions of it?
"This was what I spent most of my time on during the first week — figuring out how we could create a system for large-scale production", says Bandyopadhyay.
His solution was to set up a kind of a factory. Around the country, offices, laboratories and universities were closed down. But administrators worked lightning fast from their home offices to create exceptions to the rules, so a production unit could be set up.
Roughly 30 people were involved: technicians, researchers, and doctoral and master students. They worked in three groups that were independent and separate from each other — so that if someone in one group was infected with COVID-19, the others could continue to work.
In next to no time, large-scale production was underway. Today the mini-factory that Bandyopadhyay and his team established is producing one million tests a week. The order for 5.1 million tests for the whole of Norway has long since been delivered. The goal is to be able to produce 5 million tests a week by August.
"We haven’t signed any agreements yet with other countries, but we have received many inquiries and have sent out about 20 tests to various suppliers and public health institutes in other countries", Bjørås says.
Fastest way out of the pandemic
Since the start of the coronavirus pandemic, "testing, isolation and tracing" has been a key message from the World Health Organization, the WHO.
It wasn’t so easy to follow this advice in March, when there was a shortage of tests across the globe. And testing capacity remains a problem worldwide.
A news article in the journal Nature described a subset of efforts underway to develop new tests for the coronavirus. Norway isn’t the only country that has created its own tests, of course. Nevertheless, all these tests are needed, the article confirms. "Epidemiologists say mass testing for SARS-CoV-2 — requiring millions of tests per country per week — is the most practical way out of the current crisis", the Nature article reports.
Researchers around the world are working hard to find solutions, such as how to analyse thousands of tests simultaneously, instead of a handful or a few hundred, which is today's capacity. Others are working on making tests that don’t need expensive equipment to do the analysis, while still ensuring they are accurate enough. Many of these efforts are in the experimental stage, and not yet approved for use.
Chemicals, nanoparticles and open systems
What’s new about the NTNU test are both the magnetic nanoparticles and a specially made chemical solution.
The swab that is stuck in your nose or swabbed around your mouth is placed in this chemical solution. The solution causes the protein coat around the virus to crack. Then tiny magnetic nanoparticles are added, which bind to RNA from the virus. The nanoparticles and RNA are then extracted using a magnet and separated, so that a PCR machine can perform its analysis. The PCR machine runs a process that identifies the genetic material from the virus that causes the disease.
NTNU's COVID- 19 test proved to work well and is easy to make in large quantities. A small bottle of chemical solution and a small tube of magnetic nanoparticles are enough for 10,000 tests. Now the test may help other countries increase their test capacity, the researchers say. Perhaps it can eventually compete in the market and become a preferred test for other dangerous viruses and bacteria.
In contrast to tests from large pharmaceutical companies, the NTNU test is designed with a so-called «open liquid» system in mind. This means it can be adapted to the equipment and systems that any hospital has at its disposal.
"What a hospital uses for diagnostics is most often linked to a system provided by the supplier", Bjørås said.
"No one but the company Roche can make reagents for the tests they sell. Everything is patented and you can’t crack those systems", he said.
This was one of the big problems when the coronavirus crisis hit. The world’s pharmaceutical companies didn’t have the capacity to allow a tenfold increase in testing.
"They weren’t able to scale up in a reasonable time, and they still aren’t", says Bjørås.
"What we thought of immediately was that there are lots of open systems, such as pipetting robots and the like, which are used for research and other purposes in hospitals. So we created a solution that can be used with almost all open systems", he said.
Consequently, Norway’s hospitals have been able to use the equipment they have on hand to make the test work.
"We need to do some optimization when a robot is programmed, for example, and to handle the magnetic particles. Here, the diagnostic departments at the different hospitals have done a great job implementing the tests", Bjørås said.

Express Bureaucracy
While everything has happened at warp speed, Bjørås makes it clear that there were no shortcuts.
Two hospitals, independently of each other, ensured that the test worked — they validated it, as it is called in scientific terms — and they got the same results. Subsequently, the Norwegian Medicines Agency and the Norwegian Institute of Public Health made their assessments, before an approval for the use of the test in Norway was given on 17 April.
"We didn’t ignore the bureaucracy, it was just that it worked extremely efficiently", says Bjørås.
"It’s fantastic how efficiently things can be done. We could in theory have developed a test like this even under normal conditions, but we probably wouldn’t have because of all the regulations you usually have to follow. And then there’s the issue of financing", he said.
Bjørås said there was an amazing amount of good will in the system.
"We ordered equipment worth several million kroner in just hours. There was good will from all sides. All we had to do was to lift a finger and say we have to have this for the coronavirus test project, and then we had it that day. This was critical in ensuring the fastest possible implementation", he said.
Bjørås also credited the Coronavirus Act, which allowed the government to make decisions without getting the full approval of the Norwegian Storting.
"That meant that things could be processed faster. In this case, it meant that everything went at lightning speed", he said.

"We had been studying them for years"
When Sulalit Bandyopadhyay came to Norway from India in 2010 to take a master's degree, he never would have imagined that one day he would become part of the solution to one of the largest peacetime crises ever facing the country. It still may not have sunk in completely.
"Some people have wanted to call me a super researcher, but I said this is really not about me being a super researcher. It's about using what we've been studying for years, and it feels very useful to see that we could put it to work in this kind of situation", he said.
For the first ten days he only took breaks to sleep. His summer holidays consisted of one week off. Then there are the six-day work weeks. The group also needs Saturday to produce millions of COVID-19 tests in one week.
In fact, it was only when the media became interested that Bandyopadhyay understood what he was involved in.
"I kind of didn’t think of it as, ‘Now we’re helping Norway out of this crisis’, I thought we were just doing research", he said.
And honestly, all the media attention wasn’t that great. In the midst of the intense production, it wasn’t easy to have 70 organizations make contact and want to know everything about what they were doing and how the technology worked.
India shut down a couple of weeks after Norway. At the time of this writing, the country has almost 1.3 million recorded cases of COVID-19. But despite the shutdown, India is struggling to control the pandemic.
Maybe a COVID-19 test from NTNU can help? India is one of the countries that has contacted NTNU about using the test.
But can universities run factories?
This is not just a philosophical question. A Norwegian university has now become a major producer of a diagnostic tool. And the factory is set to continue production for at least the rest of the year.
So what happens now?
"We’re running a factory, and universities are not supposed to run factories", says Bjørås. "So we have to leave NTNU, but that is the next phase."
Work is underway to start a biotechnology company that can be based on the same technology, he says.
But why can't a university run a factory, if it works? And spend the money they make on more research?
"It's an interesting thought", Bjørås says.
"The university has used a lot of resources on developing the test, and it seems only natural that we get something back. I haven’t devoted much time to my research group lately, and haven’t gotten money for my own research. Maybe the university could take a more modern approach to this situation and earn some money that could be used to fund research? Channel some of the money that would normally go to big pharma and use it on research? Society had benefited greatly from this. It would be a much healthier use of taxpayers' money», he observes.
NTNU’s Technology Transfer Office has applied for a patent for the NTNU method. But not in order for Magnar Bjørås to make a fortune from the pandemic.
"We want to protect ourselves against anyone else being able to take the method and prevent free distribution", he says.
"A lot of the test is based on known technologies, but with important changes and adaptations in different aspects. We’ll see how the patent ends up, but the goal is for this to be made available. I want research and society to get something back from this", he said.
Essential for increasing testing
Frode Forland is the director of infection control at the Norwegian Institute of Public Health. He says that the first test in Norway was developed at the NIPH coronavirus laboratory.
"The first weeks, all samples went to us", Forland wrote in an email.
Gradually, laboratories and test capacity was built up around the country, partly based on in-house developed methods, partly based on commercial tests.
But the ability to test a lot of people requires more than just a test.
"There are a lot of different links in the logistics chain", Forland writes, including health personnel, test stations and procedures, infection control equipment, sampling equipment, transport medium, transport, laboratory personnel, extraction technology, analysis machines, interpretation of answers, and finally — sending out responses and communicating with patients.
In the first phase of the epidemic, he said, there were limitations in several of these areas.
"The magnetic nanoparticles that were developed in Trondheim were important to extract RNA from the samples. This wasn’t available on the world markets for a while, and access to an extraction medium was important for building increased test capacity in Norway", he writes.
Anne Grethe Erlandsen, State Secretary at the Ministry of Health and Care Services, wrote in an email to sciencenorway.no that "the new method was absolutely crucial for us to be able to increase test capacity significantly during the month of May, and have the ambition to test everyone with symptoms".
Testing also provides the basis for knowing when Norway can ease different control measures, and when the country may have to reinstate measures, Erlandsen wrote.
Because it is the WHO’s basic principles that continue to apply in Norway.
"Testing, infection tracing, quarantine and isolation are still the basis for the Norwegian strategy going forward to keep the epidemic under control", Forland from the NIPH said.

A piece in a larger puzzle
Not everything can be solved with nanoparticles.
Even now, when Norway has access to the equivalent of one test per inhabitant, there is still a lack of other things, such as people in the municipalities who can perform tests.
In June, a number of professors at NTNU criticized the government's strategy, in an article in the university's magazine Gemini, because the government planned only to test 1.5 per cent of a municipality’s inhabitants per week. The professors argued that a system to allow large-scale testing should be created before the autumn.
Towards the end of March, Norway was recording as many as 300 new infections daily. But then the numbers started to drop. And on 20 April, a slow reopening began. In July, the infection rates were roughtly between one and twenty new cases per day.
According to the Norwegian Directorate of Health, Norway has the capacity to test up to 5 per cent of the population within a week, should the need arise.
Universities as a part of the health service
"It feels a bit like what we did in 30 days was more important than what I have done for the last 30 years", says Magnar Bjørås.
It was his brother who took over the family farm. But growing up close to animals and nature made him Bjørås want to understand everything that had to do with biology and chemistry. NTNU was a natural choice, and the professor had his only almost-religious experience during a lecture on DNA and RNA.
" realized that this is the answer to everything. Then I thought I’ll work on this for the rest of my life", he said.
After 30 years of studying genetics, he now has had the opportunity to use this knowledge for something extremely practical and completely critical to address the crisis in which Norway and the world are still in the middle of.
However, had an EU directive from 2017 been implemented earlier, it is not certain that the university’s contribution to the fight against the coronavirus would have been legal. The EU directive on the use of medical equipment 2017/745 states, among other things, that "health institutions must be able to produce and use equipment internally to cover a specific need for a patient/patient group, provided that this is not done on an industrial scale". The directive specifies that tests may fall within the definition of medical devices.
"This is probably the result of lobbying from big industrial players", Bjørås said.
"If politicians believe that this kind of directive would provide a more secure supply, we now know that it can actually be life-threatening when a crisis arises. So there needs to be a proper discussion about this", he said.
"We now see how much expertise is found at our universities, expertise that may not be so easy for a large biotech company to replicate. This is something for the health authorities to think about. Our universities have a high level of preparedness and are an important part of the health services in Norway. Perhaps especially in a crisis situation."
This article was updated on October 7th 2020.
Translated by Nancy Bazilchuk
———