COVID-19 vaccines pave the way for new types of medication
Patients suffering from cancer, heart attacks, haemophilia or a torn meniscus might soon be treated using RNA.
“The new COVID-19 vaccines have put RNA medicines on the map in a way we could only have dreamt of a year ago,” says Sven Even Borgos.
SINTEF researcher Borgos and his colleagues are in the process of researching how RNA can be used to treat a number of different diseases, like helping the immune system kill cancer cells or repairing damaged heart tissue following a heart attack.
The research field has gained momentum since Pfizer-BioNTech’s and Moderna’s vaccines against COVID-19 were approved for use.
From ideas that have long looked exciting on paper, RNA drugs have taken a big step into the real world.
Now a lot of people, health bureaucrats and investors are realizing that it’s possible to make medicines this way.
“The pandemic might have accelerated development by a decade or more. Solid technologies can exist for quite a while before people and investors are ready for them,” says Borgos.

Cancer drugs may be the first out
The first drugs to follow in the footsteps of the new COVID-19 vaccines may be cancer treatments.
That's what Bruce Zetter, who is researching RNA as a cancer medicine at Harvard Medical School, predicts.
“There are a lot of ways you can use RNA therapy for cancer. The easiest is to use RNA to make cancer vaccines,” he says.
Zetter believes these vaccines will be on the market a couple of years earlier than they would have been otherwise.
The bureaucratic processes will now move along faster. Carrying out the necessary trials to show that the medicines are safe and getting them approved is much easier when there are already similar drugs on the market.
This means we can expect the first cancer vaccines to reach patients in two to three years, predicts the American researcher, who is also an honorary doctor at the University of Bergen.
Training your immune system
Another reason to believe that cancer drugs of this kind will appear soon is related to the German company BioNTech’s research before they started making a COVID-19 vaccine. The pharmaceutical company was already in the process of developing just such cancer vaccines.
The idea behind the RNA vaccines to prevent COVID-19 is surprisingly similar to that of the cancer vaccines.
Let's consider the COVID-19 vaccines first:
The RNA in these vaccines is a genetic recipe for the spike proteins that coat the surface of the coronavirus.
This means that the RNA triggers your own cells to make these spikes. Your immune system reacts to them before both the RNA and the spike proteins disappear again.
In this way your body learns to recognize the virus for some later time, without you having been infected.
The same thinking applies to RNA cancer vaccines:
These vaccines contain RNA through a similar recipe.
The difference is that your cells are instructed to make a completely different type of protein, which coats the surface of the cancer cells in your tumour.

The goal of both drugs is pretty similar: they are designed to make the immune system recognize and kill either the virus or the cancer cells.
That's why researchers call these cancer drugs vaccines, even though they would typically be given when you already have cancer – instead of immunizing us before we get the disease.
Haemorrhagic disease and torn meniscus
But RNA medicines may in the future offer therapies for patients suffering from other diseases as well.
“The technology could have hugely varied applications, and that is what makes it exciting,” says Borgos.
In theory, RNA could make your body create almost anything. If you’re anaemic, it can make your body produce its own EPO (erythropoietin) to stimulate red blood cell production. If you have haemophilia, RNA can trigger the body to make the substance you need to coagulate your blood.
And if you’ve torn your meniscus, RNA could in theory tell stem cells to grow new cartilage to repair it.
Body produces its own medicine
“You’re not as limited when you go in and direct one of the fundamental processes in the cell,” the SINTEF researcher explains.
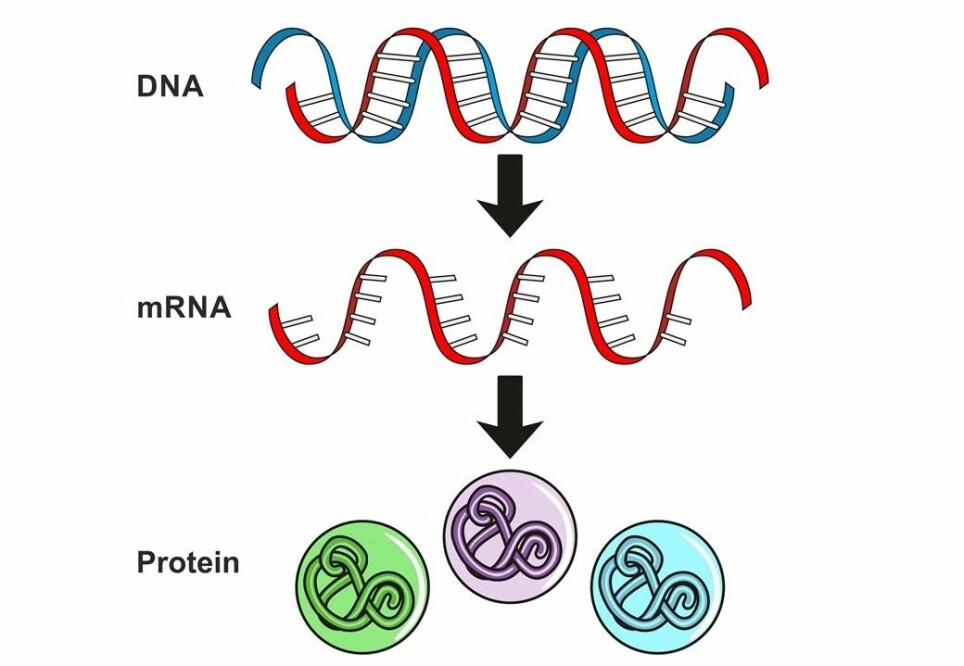
RNA consists of short-lived copies of the genetic material DNA.
The copies act as messengers that inform your cells which proteins to make at any given moment.
To be precise, we’re talking about mRNA in this case, which is a specific type of RNA where the ‘m’ stands for messenger.
“When you use mRNA as a treatment, you get the body to make its own medication. You just deliver the recipe,” Borgos explains.
Similarities with gene therapy
Some people might associate this process with gene therapy, which also involves getting the body to make its own medicine.
Zetter thinks we can look at RNA medicine as a new type of gene therapy, one that contains RNA instead of DNA.
"RNA medicine is simply a new tool we can use to correct molecular defects that cause disease," the American researcher says.
Borgos prefers not to use the term gene therapy, because some people have negative associations with gene editing.
The new type of medication differs from classical gene therapy in two ways: RNA is broken down quickly inside the body, and it cannot change our genetic material.
The SINTEF researcher believes that it will therefore be easier to gain approval for these medicines and get people to use them.
Safety always the most important consideration
But of course there are no guarantees on that.
Confidence in RNA drugs may depend on the success of COVID-19 vaccination.
And even when people have a lot of goodwill, new medicines made with the same technology aren’t guaranteed to succeed.
“Security is always the most important consideration,” says Zetter.
“First we have to continue to show that RNA is a safe therapy. And then we also need to show that every single RNA we want to use is safe.”
- Read also these articles produced by SINTEF: This is how the new mRNA Covid-19 vaccine works and How safe are the new Covid vaccines?
Promising medicine for prostate cancer
The American researcher’s projects include a new RNA treatment for prostate cancer.
The thinking behind this medicine differs from cancer vaccines, in that RNA has a different task than activating the immune system.
Instead, the medicine tells the cancer cells to stop growing. More specifically, this RNA drug contains the recipe for a protein called PTEN.
Zetter and his colleagues have shown that the treatment works on prostate cancer cells that lack this protein. But so far, the promising results are only from research on cells and mice.
It’s therefore too early to conclude that the medication will work on human cancer patients. However, the researcher believes that the trials will go much faster than they had imagined before the pandemic.
Technology to be developed in Norway
Borgos and his colleagues at SINTEF are working on an EU project called Expert.
The goal for the Norwegian researchers is to put the technology into place to be able to make new RNA drugs for testing here in Norway.
That is, they want to build the knowledge and technology needed to do everything from designing an RNA molecule to producing it and encapsulating it in lipid nanobubbles.
An advantage of these drugs is that different mRNAs are quite similar chemically.
“The chemical similarity between mRNAs means that if you’ve found a solution for one RNA, you can certainly use it for another RNA,” Borgos says.
Translated by: Ingrid P. Nuse
Reference:
M.A. Islam and others: Restoration of tumor-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA, Nature biomedical engineering 2018, https://dx.doi.org/10.1038%2Fs41551-018-0284-0
———
Read the Norwegian version of this article on forskning.no
RELATED: