
Why doesn’t all our air disappear into space?
ASK A RESEARCHER: The atmosphere may be leaking more than you think, but luckily we still have enough air down here.
Almost everyone who’s alive lives in the atmosphere – the air we breathe and live in that surrounds the whole Earth.
But how does the air stay in place? We experience the atmosphere around us as something very light and intangible.
Yet it doesn’t take off into the ethers. It’s held in place by the same force that holds everything else down on Earth: gravity.
“Earth is big and heavy,” says Bjørn Samset. He is a physicist and senior researcher at the Cicero Center for International Climate Research in Oslo.
“These gases can’t escape as long as there is earth that holds them in place.”
Earth was created from a whole range of different elements well over 4 billion years ago. In this process, the elements’ molecules settled from the heaviest on the inside to the lightest outside, and everything is held in place by the same gravitational force.
The lightest molecules are suspended somewhat like a thin veil, or very thin liquid, around the planet.
Samset points out that all these molecules in the atmosphere would have accumulated in layers, from the heaviest at the bottom to the lightest at the outermost edge, assuming no mixing took place in the atmosphere. But in the real world, the sun adds a huge amount of energy to the atmosphere, and there's a lot going on up there.

A tiny bit of the air actually escapes into space. Around 90 tonnes of the atmosphere disappears into space every day, according to the European Space Agency.
This sounds like a lot, but it's just a tiny part of the atmosphere.
“It would probably take more than 150 billion years before the atmosphere disappeared this way,” says Samset.
Why some of the oxygen escapes is a complicated question, but we’ll return to that later in the article.
But first: What’s actually in the air?
The air that doesn’t escape
Air is made up of a whole range of different gases, but it consists of as much as 80 percent nitrogen. Vitally essential oxygen makes up around 20 percent, along with smaller amounts of argon, carbon dioxide, helium, hydrogen and other substances.
But this is where our intuition about the world trips up slightly. We experience rocks as something heavy and tangible, while air is something fundamentally different. The philosophers of antiquity and other parts of the world came up with the four elements earth, fire, air and water as having qualities that are unique and different from each other.
But that's not how the world works. Everything is made up of elements, and these elements have mass and weight.
“There’s really no difference between a nitrogen molecule and a rock,” says Samset. “Or as Yoda says, it’s ‘only different in your mind.’"
“For a nitrogen molecule to escape from Earth, it has to get energy from somewhere.”
For either a nitrogen molecule or a rock is to rise above Earth's deep gravitational field, energy needs to be supplied. When we send objects into space, we use rockets to counteract gravity.
This requires a lot of energy, which explains why the rockets we use are so large and need so much fuel. To escape Earth's gravity from ground level, you have to ascend at over 40 000 kilometres per hour.
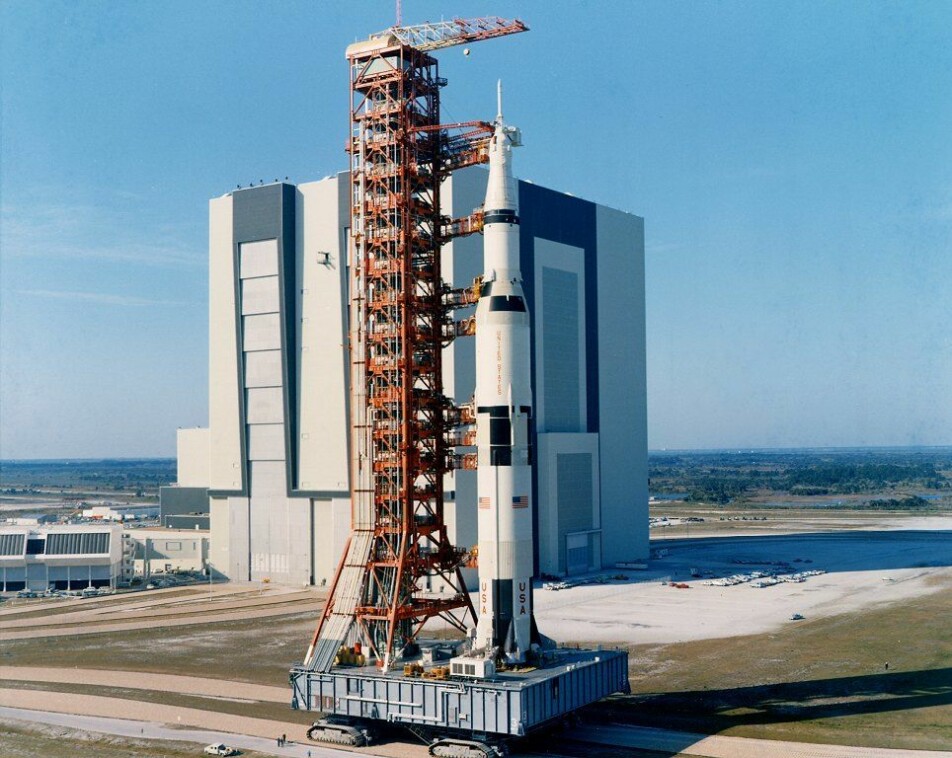
A Saturn V rocket carried a lunar rover, the spacecraft and three astronauts to the moon, with a total maximum weight of 130 tonnes. But to launch it into space, the entire rocket weighed 2 800 tonnes – most of which was fuel, according to NASA.
A molecule with nitrogen is much lighter than the rocket load, but energy must still be supplied for it to escape.
And this can happen in several ways.
Light elements
Some substances, like helium and hydrogen, are lighter than air. These gases rise into the atmosphere, yet this does not mean that they disappear into space when you puncture a helium balloon, for example.
A small amount of helium is scattered in the atmosphere, and the outer layer of the atmosphere – the exosphere – contains extremely thinly scattered helium and hydrogen.
The sun supplies energy, which warms the atmosphere and causes atoms and molecules to move more, and this energy can eject matter. Hydrogen and helium are also added to the atmosphere from the sun.
The molecules in a gas move and crash into each other all the time, and sometimes molecules from these and other gases get such a powerful jolt that they’re ejected from the atmosphere. They reach escape velocity, and then break free of Earth's gravitational pull.
“Up there, gravity is weaker than at ground level,” says Samset.
“Some of these jolts are so strong that gases manage to escape. It's almost like a random rocket.”
But this "random rocket" effect accounts for only a small part, around 10 to 20 percent, of what actually escapes from the atmosphere.
Exactly why the rest disappears is a mystery.
Why does oxygen disappear?
Oxygen and nitrogen molecules shouldn’t be hurled out of the atmosphere, because they’re too heavy, Jøran Moen says.
Moen has been researching our leaking atmosphere with the help of small research rockets fired from Svalbard, where he now works as a director at the University Centre in Svalbard (UNIS).
An oxygen atom is many times more massive than a helium atom.
Yet there is a current of oxygen, helium and hydrogen that disappears over Earth's magnetic poles – an atmospheric leak. The research rockets are fired through the leaks to see what is really going on here.
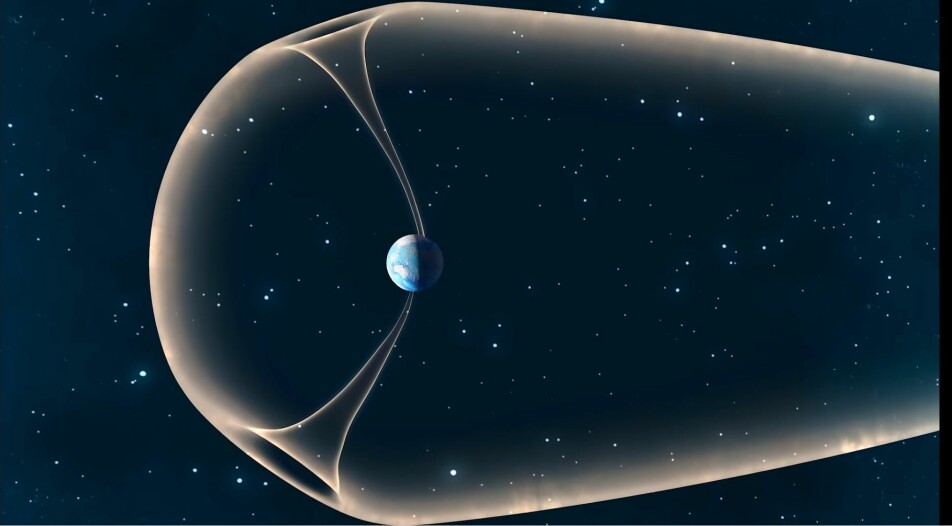
These leaks are almost like plumes of atoms protruding beyond the two magnetic poles of Earth. And here the molecules are so heavy that it takes a lot of energy to get them out.
The air in these plumes is very dense compared to the space around them. Moen describes how satellites suddenly experience much greater resistance when they pass through the plumes, because the density of gas is much greater right there.
Moen explains that they don’t know exactly where all this energy comes from, but it can be traced to the solar wind – the stream of charged particles that continuously hit the atmosphere. These particles also create the Northern Lights – a phenomenon associated with the atmospheric plumes.
10 000 degrees
“The charged particles create a strong warming of the atmosphere – up to 5000 to 10 000 degrees Kelvin,” says Moen.
These temperatures approach 10 000 degrees Celsius.
However, this heating does not generate enough energy to eject the heavy atoms and molecules.
Moen says that this is probably related to the electrical coupling that takes place between Earth's magnetic field and the solar wind that hits the magnetic field.
The coupling adds energy and has a potential for strong heating of the atmosphere. The heated atmosphere particles can follow the magnetic lines in Earth's magnetic field and are ejected over the magnetic poles, forming these plumes.
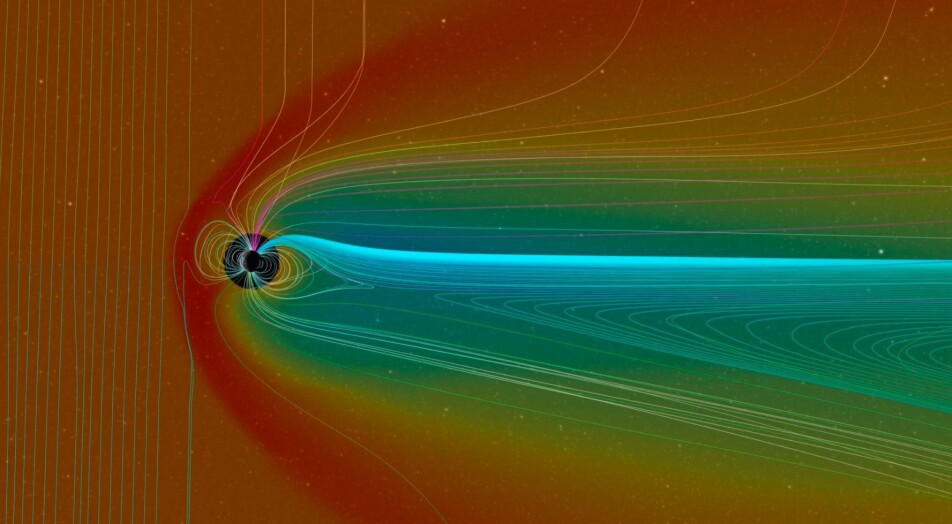
“But we haven’t found the precise heating mechanisms,” says Moen. He says that this is ongoing work.
Earth's magnetic field actually protects the atmosphere – and all of us who live here – from the charged particles that come with the solar wind. Without the magnetic field, the atmosphere would slowly but surely be peeled away and thinned out. But this would take a very long time, according to Live Science.
Mars probably had a powerful magnetic field in the early days of the solar system, but this field stopped working billions of years ago, according to the BBC. At that time, Mars had a much thicker atmosphere, which was greatly reduced by the solar wind. This is one of the reasons why Mars is the desert planet we know today.
So even though Earth's atmosphere is leaking a little, it won’t run out for a long, long time.
And long before all the air escapes into space, Earth will be uninhabitable for us due to the expanding sun and warming temperatures.
Translated by Ingrid Nuse.































