Quantum mechanics is 100 years old. Without it, we wouldn't have this technology
Transistors have revolutionised society, says researcher.
Quantum physics describes how the smallest building blocks in the universe behave and interact.
The UN has designated 2025 as the International Year of Quantum Science and Technology. The year will mark 100 years since researchers first began working with the development of quantum mechanics.
Jacob Wüsthoff Linder thinks it's great that this is being celebrated. He is a professor at NTNU's Department of Physics.
“It's not just about quantum mechanics giving us an accurate understanding of how nature works, but that it has also led to concrete applications,” he says.
But why is 1925 considered the start of quantum mechanics?
A significant equation
“One could perhaps argue that the first seeds of the quantum mechanical theory were sown a bit earlier in the 20th century,” says Linder.
Discoveries made at that time suggested that energy could be divided into packets, or quanta, without smooth transitions.
Max Planck, Albert Einstein, and Niels Bohr made important contributions.
“But it's quite okay to say that 100 years ago, quantum mechanics really began to take off,” says Linder.
One of the things that happened at that time was the development of an equation – the Schrödinger equation.

“This is the starting point for much of the physics described in the theory of quantum mechanics,” he says.
Both wave and particle
What did this equation reveal?
Quantum physics breaks with how we usually perceive the world, says Linder.
“We're used to thinking of things like a football or a glass as something solid, or as a composition of many particles. In quantum mechanics, matter can be described as waves. That's quite different,” he says.
The smallest things, like electrons and atoms, behave both as waves and as particles.
“The Schrödinger equation describes the wave properties that matter or mass has,” says Linder.
This leads to some strange consequences.
Cannot be measured precisely
In the classical understanding of physics, the universe is predictable.
“We can calculate very precisely where a particle is at any time, where it's moving, what speed it has, and so on,” says Linder.
In quantum mechanics, the properties of particles are more uncertain.
“If we know the position of the particle, quantum mechanics says that there's a great uncertainty about its speed,” he says.
This is Heisenberg's uncertainty principle.
"According to quantum mechanics, not all properties of masses or particles can be precisely determined,” he says.
So how has this understanding of the strange behaviour of electrons, atoms, and light been useful over the last 100 years?
1. Lasers
One of the first things quantum mechanics was used for was with lasers, says Linder.
Again, it was Einstein who was early on the scene.
“He discovered something called stimulated emission. That's the principle a laser is based on,” says Linder.
It was not until the 1940s and 1950s that people began to explore how this theory could be applied. It involves the idea that electrons in atoms can only have specific energy levels.
If the atoms are exposed to light with just the right frequency, the electrons jump to a lower energy level. They then emit energy in the form of light with the same frequency as the incoming radiation.
“This creates a kind of self-reinforcing effect. You irradiate a material with light and generate more light with exactly the same properties,” he says.
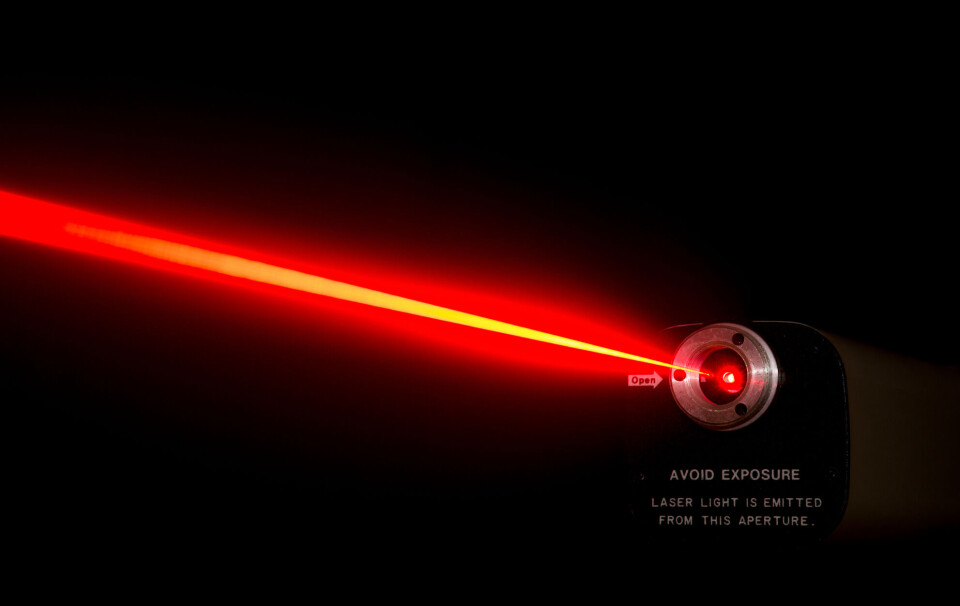
Laser light becomes intense, monochromatic, and travels in a sharply defined direction.
Lasers are used for many different things today – such as making precise measurements, treating skin and eye conditions, and in the military.
“A more everyday application is laser light used to scan groceries at the store,” he says.
Lasers are also used in fibre cables to transmit data traffic over the internet.
2. Transistors
The first transistor came in 1947. They became commercially available in the late 1950s.
Most viewed
“This is probably the application of quantum mechanics that has had the greatest impact on society. Computers, mobile phones, and many other electronic gadgets use transistors,” says Linder.
He explains that this is a small circuit that allows you to amplify and control electrical signals.
Quantum mechanics tells us that electrons in materials exist at specific energy levels.
“There are certain energies that electrons are forbidden to have. This is called a gap. In a transistor, the forbidden energy levels are used to control how much current passes through,” he says.
Without quantum mechanics, today's technology would look very different.
“Computers would still be the size of rooms,” he says.
3. MRI machines
Magnetic Resonance Imaging (MRI) machines are used to take detailed images of tissue in the body. MRI does not use X-rays and is therefore easier on the body. The method can also provide better images of certain types of tissue.
“MRI machines use quantum mechanics through a property called spin,” says Linder.
An MRI machine creates a strong magnetic field that causes hydrogen atoms in the body to align in a specific way. Radio waves are emitted that affect the atoms and makes them to twist. The atoms return to their original position and release energy.
This is used to produce detailed images of different tissues.
4. LED lights
“It wasn't that long ago that we used light bulbs that got very hot. If you touched the bulb, you could burn youself,” says Linder.
Today we have LED lights, which are much more energy efficient. The electricity used in LED lights mainly goes toward producing light, with very little wasted as heat.
“Once again, it's quantum mechanics that makes LED lights possible,” he says.

It goes back to electrons and their allowed energy levels.
“An LED light consists of two regions,” he says.
One region contains lots of electrons that have high energy. Another region has no electrons and there many empty spaces around the atoms.
“When you turn on the power, the high-energy electrons start moving into the region with all the empty spaces,” he says.
The electrons settle into lower energy levels first, and when they drop down in level, they release the remaining energy they had as light.
What can it be used for in the future?
It's hard to predict the future, says Linder, but there is clearly a lot of interest in quantum computers.
“Several of the major players – IBM, Microsoft, and Google – have invested a lot of resources in trying to be the first to create a useful quantum computer,” he says.
Computers based on quantum mechanical principles have been developed. So far, they are not particularly useful.
“There's still a long way to go before we have quantum computers that are significantly better at doing certain things than the best conventional computers we have today,” says Linder.
But quantum computers could become big in the future, if they can be made.
Linder concludes by encouraging people to learn a bit about the basic ideas of quantum mechanics.
“It's a bizarre, but also very exciting way to look at the world,” he says.
———
Translated by Nancy Bazilchuk
Read the Norwegian version of this article on forskning.no
Related content:
Subscribe to our newsletter
The latest news from Science Norway, sent twice a week and completely free.